Borates are highly recommended for pools. Adding borate is a one-time thing. Borate does not decompose, get used up or expire. Borate is only lowered by water loss – splash out, drag out, leaks, and filter backwashing , or draining. So, you add it once, and unless you are losing a lot of water, you don’t need to add it again until you drain the pool and refill it.
Benefits of Using Borate
- Keeps the pH of the water from going up (pH buffer against rises in pH)
- Helps prevent algae (it is an algaestat, not an algaecide)
- Lowers chlorine demand (preventing algae lowers the need for chlorine)
- Helps protect HOCl (hypochlorous acid – the killing form of free chlorine) from UV destruction
- Water feels softer and silkier
- Water looks clearer and sparkles
Except for the last two points, these are not just manufacturer or sales claims but proven benefits when the concentration of borate in the water is 50 ppm. There is no published evidence in physical science for a mechanism of enhanced blue color or sparkle or for a silkier or softer feel to water. However, these anecdotal claims still persist in manufacturer’s literature, service tech observations and pool owner reports.
Pools equipped with SWG (salt water chlorine generator) or ECG (electronic chlorine generator) will benefit from a level of borate of 50 to 70 ppm, however, the US EPA and NSF do not recommend levels more than 50 ppm. More later.
Using borates with an SWG or ECG
Borate is a buffer against pH increase so this means less calcium carbonate scaling on the hydrogen gas (H2) generation plate (negative electrode) of the SWG. That should be it’s only side effect and it is a benefit. Also, keeping 50 ppm of borate lowers the recommendation that free chlorine should be 7.5% of CYA to 5% of CYA. This is a minimum of 33% less chlorine.
Borate as a pH Buffer
Buffering is when the water resists changes in pH when acid or base are added to it. The measure of bicarbonates (HCO –) and carbonates ( CO –2) is called total alkalinity. However, most of the total alkalinity in pool water is made up of bicarbonate.
Cyanuric acid (CYA) prevents UV destruction of chlorine in the water. It is added separately as chlorine stabilizer or conditioner or it is added when using trichlor tabs. The tabs are about 50 percent CYA and the CYA level continuously builds up in the water. CYA is a buffer against pH decrease.
Adding 10 ppm of free chlorine to the water using trichlor increases CYA by 6.0 ppm. Therefore, the increase to CYA requires a higher free chlorine level to kill bacteria and algae.
Together total alkalinity (TA) and cyanuric acid (CYA) make up the buffering system in the water against pH decrease.
 Adding borate to the water prevents the pH from rising so borate is a pH buffer too. Keeping total alkalinity at 90 ppm, cyanuric acid at 30-50 ppm and borate at 50 ppm, makes an ideal overall buffering system against up or down pH changes.
Adding borate to the water prevents the pH from rising so borate is a pH buffer too. Keeping total alkalinity at 90 ppm, cyanuric acid at 30-50 ppm and borate at 50 ppm, makes an ideal overall buffering system against up or down pH changes.
If the pool has a SWG (Salt Water Chlorine Generator), borate is especially helpful because in the process of making chlorine from salt, SWGs produce a high pH. This requires acid to be added to lower the pH but acid also low- ers alkalinity. The result is high pH and low alkalinity. This requires addition of sodium bicarb to raise alkalinity.
Using borate as a buffer with an SWG slows the rate of pH rise. The pH may still rise, but not as dramatically. So you will need to add acid less frequently. Bicarb will not be needed as often either. Ideal borate level with SWG is 50-70 ppm borate, however, the EPA maximum is 50 ppm with a level of concern of 100 times that.
Borate as an Algaestat
Many articles claim that borate is an algaecide and that borate works as an algaecide by removing CO2 from the water. Algae require CO2 to carry out photosynthesis so removing CO2 would kill algae. Logical but wrong.
Algae are carbon-based life forms. The fact is that algae can get plenty of carbon or carbon dioxide (CO2) from the carbonates (CO –2) and bicarbonates (HCO –) in the water (total alkalinity).
Borate does NOT remove any CO2 from the water and even if it did, the next time soda ash or sodium bicarb is added, CO2 is added. In addition, CO2 from our atmosphere is continuously dissolving into the water because CO2 in the water is at equilibrium with CO2 in the atmo- sphere and that would be enough to support algal growth.
So how does borate prevent algae?
Borate disrupts cell wall development, metabolism and cell division. So borate is good at preventing algae (algaestat- ic) rather than killing algae (algicidal). You will need less chlorine (have a lower chlorine demand) when using borates because you are preventing algae. Some manufacturers claim 50% reduction in chlorine consumption.
The Chemistry of Borates
OK, if you really don’t care about the chemistry of borates, skip to the “Borate Products and How to Use Them” section below.
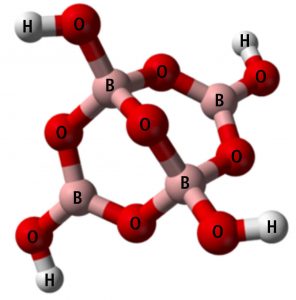
Boric acid, formula B(OH)3 or H3BO3, is technically a Lewis acid. It does not release a hydrogen ion (H+) like most acids, but it can acquire an OH– from the water according to this equation:
B(OH)3 + H2O ↔ B(OH)4- + H+
Boric acid plus water yields borate ion plus hydrogen ion
The final effect is that H+ (hydrogen ion) increases which lowers pH (remember, pH is a measure of H+ in water and the more H+, the lower the pH).
Borax, formula Na2B4O7•10H2O , is a salt of boric acid. When added to water it dissociates into sodium ion (Na+) and borate ion B(OH) – .
The above boric acid–borate ion equation is reversible and in equilibrium as seen by the arrows going both directions. In solution, there is an equilibrium between the boric acid and the borate ion. At normal pH values in pool water, (7.4-7.6), the predominant form is boric acid. However, the benefit of keeping the pH from rising (pH buffering) is due to the borate ion and is more evident at higher pH values or as the pH rises.
Here we see that it takes more and more soda ash to increase pH with borate in the water as the pH becomes higher. It takes a lot less soda ash to raise pH near 7.0. This clearly shows the buffering capacity of borate in keeping the pH from rising.
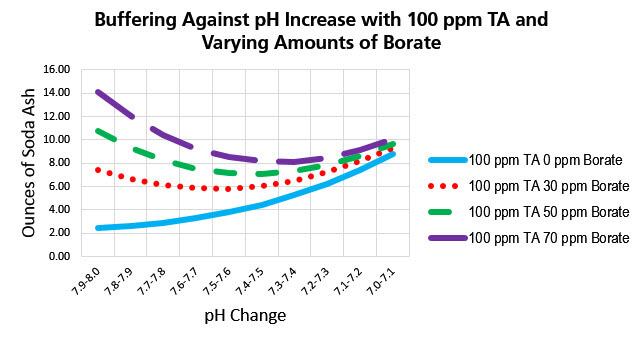 Borate Products and How to Use Them
Borate Products and How to Use Them
Read the information below about borate products and decide which product and procedure will work best for you. We recommend using boric acid because the pH adjustment is minor and the overall cost is the least expensive. However, finding boric acid in your area may be difficult.
Boric Acid
The chemical formula is B(OH)3 or H3BO3, same thing. Boric acid is sold at home centers, hardware and gardening stores. It is often sold in nurseries and garden stores as a bug killer.
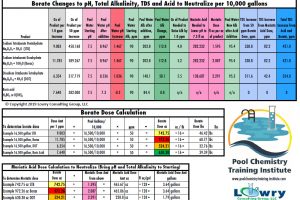
Adding 76 oz or 4.75 lbs of boric acid per 10,000 gallons of water will provide 10 ppm of borate.
Boric acid is a weak acid and has a pH of 3.8-4.8. It will not lower pool water pH by much. Most of the time the pH drop is only about 0.2 for a 50 ppm dose. Therefore, you can add boric acid without having to add muriatic acid like when adding Borax or sodium tetraborate pentahydrate.
Also, if you have metals in the water or have had metal staining, boric acid will not cause additional staining, deposits or precipitation. No problem with high pH and alkalinity either. In fact, you just add it and in about 12 hours or so, you test pH and alkalinity and adjust them if necessary.
Calculate Dose of Boric Acid
Amount Needed per 10,000 gallons per 10 ppm = 76 oz Pool Volume Factor = Pool Volume ÷ 10,000
Dose Factor = Desired Increase ÷ 10 ppm
Boric Acid Dose Amount = Amount Needed × Pool Volume Factor × Dose Factor
Example:
16,500 gallon pool and 50 ppm Borate
Amount Needed per 10,000 gallons per 10 ppm = 76 oz Pool Volume Factor = 16,500 ÷ 10,000 = 1.65
Dose Factor = 50 ppm ÷ 10 ppm = 5
Boric Acid Dose Amount = 76 oz × 1.65 × 5 = 627 oz 627 ÷ 16 oz/lb = 39.19 lbs
The CO$T
Finding boric acid in large containers may be difficult in your area. It is sold as a pesticide and in small amounts in land- scaping companies, nurseries, gardening stores and home centers. However, we did find a source that ships nationwide. The website for boric acid and other borate products is here:
http://www.dudadiesel.com/search.php?query=boric&gclid=CLyEvaWM2rcCFQyk4AodPwcAmA.
The site is Duda Diesel and they sell boric acid for $25.95 for 10 lbs, $46.99 for 25 lbs and $55.99 for 45 lbs plus applica- ble tax with free shipping from Alabama. The powder version works better than the granules.
Procedure
- Turn on the pump and leave it on for the next 24
- Measure or weigh out the dose of Boric Acid. Break up any lumps with a scoop or other
- Using a scoop or plastic bowl broadcast the powder into the water while walking around the
- If any powder accumulates on the pool bottom, use a pool brush to help it
- Wait 12
- Test the pH and alkalinity of the
- Adjust as needed.
Borax (not Boraxo)
The chemical name is sodium tetraborate decahydrate, formula Na2B4O7•10H2O . This is probably best known as 20 Mule Team Borax™. It is often found in grocery stores in the laundry aisle. Boraxo™ is soap or laundry detergent. You don’t want this.
Adding 118 oz or 7.4 lbs of Borax per 10,000 gallons of pool water will provide 10 ppm borate.
Also, 20 Mule Team Borax™ is sold in boxes that contain 4 lbs 12 oz (76 oz or 4.75 lbs). So as an approximate amount, you will need 2 boxes per 10,000 gallons per 10 ppm. This will be slightly more than 10 ppm but we don’t need to be exact as the pool gallons may not be that accurate either. Close is OK and a borate level that is more than 50 ppm is actually better.
Borax has a pH of about 9.2 and it will raise the pH of the pool water when added. This has two potential problems.
First, if there are any metals in the water (copper, iron or manganese) there is a possibility of causing a stain. Metals may be dissolved in the water and may be at the saturation point for that pH, alkalinity and hardness. When the pH is raised by adding Borax the metals may precipitate out causing a stain. So if you have had problems with metal stains in the past or if you are using well water, you should consider testing the water for metals and then using a metal remover, stain inhibitor chemical (called sequestering agents) or using another borate-containing product such as boric acid or a borate pH neutralized product instead of Borax (see below).
Second, to offset the high pH of the Borax you will need to add muriatic acid (31.45% HCl). The amount needed is 3 gallons for 10 boxes of Borax or 2 fl oz of muriatic acid for each 1 oz of Borax added.
In addition, before adding the Borax, the alkalinity should be about 80 ppm and the pH should be about 7.4 or less. The Total Alkalinity must be lower than 140 ppm and the Hardness must be less than 350 ppm before adding Borax. Adjusting these levels may or may not be needed. Test the water to be sure and make the adjustments.
Calculate Dose of Borax
Amount Needed per 10,000 gallons per 10 ppm = 118 oz Pool Volume Factor = Pool Volume ÷ 10,000
Dose Factor = Desired Increase ÷ 10 ppm
Borax Dose Amount = Amount Needed × Pool Volume Factor × Dose Factor
Example:
16,500 gallon pool and 50 ppm Borate
Amount Needed per 10,000 gallons per 10 ppm = 118 oz Pool Volume Factor = 16,500 ÷ 10,000 = 1.65
Dose Factor = 50 ppm ÷ 10 ppm = 5
Boric Acid Dose Amount = 118 oz × 1.65 × 5 = 973.5 oz
973.5 ÷ 16 oz/lb = 60.84 lbs or 13 boxes of Borax (4.75 lbs each)
Calculate Muriatic Acid Dose
Amount of Muriatic Acid Needed = 1.0 fl oz per 2 oz of Borax or 3 gallons per 10 boxes of Borax
Example:
973.5 oz of Borax ÷ 2 oz of Boraxo per 1 fl oz muriatic acid = 486.75 fl oz
486.75 fl oz ÷ 128 fl oz per gallon = 3.8 gallons muriatic acid or nearly 4 gallons 3 gallons per 10 boxes of Borax so 13 boxes ÷ 10 = 1.33 × 3 = 4 gallons
The CO$T
Currently, 20 Mule Team Borax™ sells for $4.17 per 76 oz (4.75 lbs) box. So 13 boxes is $54.21 plus tax at about 8% which is $4.34 or about $58.50. Muriatic acid sells for $4.99 per gallon, so 4 gallons is about $20.00 with tax. The total is about $78.50 for the 16,500 gallon pool in this example.
Procedure
- Test water for metals. If present, remove metals or use a stain inhibitor before
- Total Alkalinity must be less than 140 ppm, Hardness less than 350 ppm and pH less than 7.4. Adjust if
- Turn on the pump and leave it on for the next 24
- Empty about ½ of the Borax dose into a large bucket or Break up any lumps with a scoop or other tool. (In the example we would empty 6 or 7 boxes.)
- Using a scoop or plastic bowl broadcast the powder into the water while walking around the
- If any powder accumulates on the pool bottom, use a pool brush to help it
- Now add ½ of the Muriatic Add it near a return line so it mixes well. In the example, we would add 2 gallons of muriatic acid.)
- Wait about 20-30 minutes
- Add the remaining Borax in the same (In the example we would add 6 or 7 boxes.)
- Add the remaining Muriatic Acid as above. (In the example we would add 2 gallons of )
- Wait 2
- Test the pH of the If it is very high, the test will turn purple. Add 1 quart of muriatic acid.
- If the pH is light to dark red, add ½ quart (1 pint) of muriatic
- Wait 2
- Test the pH again. Continue testing and adding 1 pint of acid each time until the pH is 4-7.5.
- Wait at least 12 hours and retest the pH and Total Adjust as needed.
- The acid addition will lower alkalinity to about 90 ppm. However, this will make the pH near 3.You will need to aerate and cause turbulence to raise only pH. Do not use soda ash to raise pH from 6.3 to 7.5 be- cause it will raise TA to more than 300 ppm. Aeration and turbulence raises only pH with no change to TA.
The directions and procedures for adding sodium tetraborate pentahydrate to a pool are the same as for adding Borax above. The difference is that you will need less of this chemical because it contains half as much water – pentahydrate (5 H2O) versus decahydrate (10 H2O).
Adding 90 oz or 5.6 lbs of sodium tetraborate pentahydrate per 10,000 gallons of water will provide 10 ppm.
Just like Borax, sodium tetraborate pentahydrate has a high pH of about 9.2 so it will require the addition of muriatic acid. To offset the high pH you will need to add muriatic acid (31.45% HCl). The amount needed is 1 gallon for each 4 lbs of sodium tetraborate pentahydrate or 1 fl oz of muriatic acid for each 1.6 oz of sodium tetraborate pentahydrate.
Calculate Dose of Sodium Tetraborate Pentahydrate
Amount Needed per 10,000 gallons per 10 ppm = 90 oz Pool Volume Factor = Pool Volume ÷ 10,000
Dose Factor = Desired Increase ÷ 10 ppm
Borax Dose Amount = Amount Needed × Pool Volume Factor × Dose Factor
Example:
16,500 gallon pool and 50 ppm Borate
Amount Needed per 10,000 gallons per 10 ppm = 90 oz Pool Volume Factor = 16,500 ÷ 10,000 = 1.65
Dose Factor = 50 ppm ÷ 10 ppm = 5
Boric Acid Dose Amount = 90 oz × 1.65 × 5 = 742.5 oz
742.5 ÷ 16 oz/lb = 46.4 lbs
Calculate Muriatic Acid Dose
Amount of Muriatic Acid Needed = 1.0 fl oz per 1.6 oz of sodium tetraborate pentahydrate
Example:
742.5 oz of sodium tetraborate pentahydrate ÷ 1.6 of sodium tetraborate per 1 fl oz muriatic acid = 464 fl oz
464 fl oz ÷ 128 fl oz per gallon = 3.6 gallons muriatic acid.
The CO$T
Currently, sodium tetraborate pentahydrate costs about $50.00 for 20 lbs or $2.50 per pound. In the example, we need 47 lbs which would cost $117.50. Muriatic acid sells for $4.99 per gallon, so 3.6 gallons is about $18.00 with tax. The total is about $137.00 for the 16,500 gallon pool in this example.
Borate Products pH Neutralized
There are a few products available that combine the borate chemicals with pH neutralizing chemicals and perhaps some metal inhibitors or other additives so all you have to do is add them. They are convenient and easy to use but as you might have guessed, they are more expensive than other borate products. The two products are ProTeam Supreme Plus and Salt Support. The ProTeam Supreme Plus is 45 lbs and sells for $157.07 from Amazon plus shipping. The cost per pound is about $3.50. The use directions for this product are 3.35 lbs per 1,000 gallons. So using our example of 16,500 gallons, we would need 55.3 lbs and at $3.50 per pound that is $193.55 to add 50 ppm of borate to a 16,500 gallon pool. Compare that to about $60.00 for Borax or boric acid. Convenience has its cost.
In Summary
| Cost Comparison of Borate Products | |||||||
|
Product |
Cost per oz (cost per lb ÷ 16 oz per lb) |
Dose Amount (oz) Need- ed for 50 ppm in 10,000
gallons |
Dose Cost |
Muriatic Acid Cost per oz |
Amount Needed to Neutralize Dose (oz) |
Neutral- ization Cost |
Total Cost for 50 ppm in 10,000 Gallons |
| Borax (sodium tetraborate decahydrate) | 0.060 | 590 | $ 35.40 | 0.039 | 295 | $ 11.50 | $ 46.90 |
| Sodium tetraborate pentahydrate | 0.156 | 450 | $ 70.20 | 0.039 | 225 | $ 8.78 | $ 78.98 |
| Boric Acid | 0.121 | 380 | $ 45.98 | 0.039 | 0 | $ – | $ 45.98 |
| Neutralized Borate | 0.219 | 536 | $117.38 | 0.039 | 0 | $ – | $117.38 |
| Product | 10 ppm/10,000 gal amount | PPM Borate Increase
per 10,000 gallons |
1 Measuring Cup
Weight |
| Borax (sodium tetraborate decahydrate) | 118 oz (7.4 lbs) | 1.36 ppm/1 lb | 8.35 oz |
| Sodium tetraborate pentahydrate | 90 oz (5.6 lbs) | 1.78 ppm/1 lb | 9.18 oz |
| Boric acid | 76 oz (4.8 lbs) | 2.10 ppm/1 lb | 7.18 oz |
There is a big difference in the weight of 1 measuring cup of these products and a big difference in the ppm of borate provided by 1 pound. It is better to weigh borate products rather than using a scoop or measuring cup.
Regardless of which product you use, we highly recommend using borates in pool water. It will make maintaining pools easier with fewer problems and better water quality.
Cost is not the only consideration in choosing a borate product. Availability, ease of use, convenience, muriatic acid use, storage, handling, personal safety, time and effort are all considerations.
Boric Acid or Sodium Tetraborate Pentahydrate?
If you use sodium tetraborate pentahydrate (let’s just use tetraborate) you will increase pH and total alkalinity in addi- tion to raising borate in the water. The pH goes really high (near 9.0) and the TA more than doubles. This requires adding a large amount of acid to lower TA back to a target of 90 ppm which will lower pH much lower than 7.0. Then you need to aerate and create turbulence and splashing to raise only the pH. If you add soda ash to raise the pH back up, you will also raise alkalinity which makes TA too high.
If you use boric acid to increase borate in the water, it will only lower pH by 0.2 and have no effect on total alkalinity.
Let’s do an example:
Pool volume: 15,000 gallons pH: 7.5
TA: 90 ppm
CH: 350 ppm
CYA: 50 ppm
Goal: Increase borate (boron, chemical symbol B) by 50 ppm
Sodium tetraborate pentahydrate dose: 684.7 oz or 42.8 lbs
New pH: 8.97 and pH increase is 1.47 from 7.5 to 8.97
New TA: 204 and TA increase is 114 ppm from 90 ppm to 204 ppm
Acid dose required to lower TA from 204 to 90 ppm: 3.47 gallons of muriatic acid (31.4% HCl)
New pH: 6.32 and pH decrease is 2.65 from 8.97 to 6.32
New TA: 90 and the TA decrease is 114 ppm from 204 ppm to 90 ppm
But pH is now 6.32. You must aerate and make turbulence and splashing to raise only pH to 7.5.
If you try to raise pH with soda ash from 6.32 to 7.5 you will increase TA from 90 ppm to 312 ppm.
This is why boric acid is better.
Boric Acid dose: 572.73 oz or 35.8 lbs New pH: 7.3
New TA: 90 ppm
Done or you could aerate and cause turbulence to raise only pH from 7.3 to 7.5. Much easier.
“I Can’t Afford $50 or $60 Per Pool”
We hear this comment a lot. Using borate is an up-front cost but it will save you money and time very quickly. You will be using 33% to 50% less chlorine. With 50 ppm borate, the recommended free chlorine level is 5% of CYA instead of 7.5%. You will be using less acid because the pH will not be going up as much. If you lower CYA to 50 ppm, the chlorine savings will be even greater because 5% of 50 ppm is less than 5% of 100 ppm. You will have less problems. You will not need to shock or superchlorinate your pools any more. You will not need algaecides. You will not have to spend as much time at each pool which saves money. You may even be able to take care of one more pool per day. You also may be able to buy boric acid in bulk if you are going to add it to many pools.
Testing for Borate
You will need to buy some borate test strips. Over time with water loss from backwashing, filter cleaning, splash out, drag out and other water loss, (but not evaporation), you will need to bring the level back up to 50 ppm or more. The only way to do this is to know the borate level in the water. Borate test strips are made by LaMotte, ITS, Hach and Taylor.
Check the borate level about once a month. Whenever the level gets near 30 ppm, you should add sufficient borate to bring it back to 50-60 ppm. It is no problem and there are some benefits from higher levels but never more than 70 ppm.
Safety
In spite of the fact that borate is used in washing clothes and has been used for more than 100 years, you should follow all label directions, precautions and wear suitable protective equipment when handling and storing these chemicals.
Muriatic acid is especially dangerous and requires handling with prudent caution.
Borate Toxicity
Several common fruits and vegetables typically contain 160-300 ppm boron. Sea water contains about 4.5 ppm boron and some saline lakes contain above 300 ppm boron.
The Acute LD50 is the amount of a chemical necessary for a lethal dose of 50% of the test subjects. It is usually expressed as grams/kilogram (g/kg). Acute oral LD50 for boric acid in laboratory animals is in the range 2.5-5 g/kg; about the same as table salt (NaCl: LD50 3-4 g/kg).
For an individual weighing 70 kg (150 lbs), this would indicate more than 150 g or 5.3 oz of pure boric acid for lethal effect. From a study of fewer individuals, dogs may be about 4-fold more sensitive. From accidental poisonings in humans, minimum oral lethal doses of boric acid have been estimated to be in the range of 5-20 g for adults, 3-6 g for children.
To obtain a 5 g dose of boric acid from a swimming pool at 50 ppm boron it would be necessary to swallow 20 liters (about 5.25 gallons) of pool water.
Dogs and cats (and people) should not drink pool water because it contains many chemicals that are not good for our pets. Provide fresh water outside and change it regularly. It is fairly easy to teach pets not to drink from the pool. A good tip is to put a chair on the top step of the pool access. Dogs usually stand on the top step to drink. Blocking access helps.
In the 2008 Tolerance Reassessment Eligibility Decision (TRED), the U.S. EPA (2008b) selected a no observable adverse effect level (NOAEL) of 8.8 mg/kg-day (as boron) from a chronic toxicity study in dogs in order to assess the margin of exposures for various residential uses of boron (including its use in swimming pools). Each margin of exposure (calculated by dividing the NOAEL of 8.8 mg/kg-day by the estimated exposure for a specific residential use) was compared against a level of concern (LOC) of I00.
It should be noted that a concentration of 30-50 mg/L (as boron) is a common recommendation, which can exceed the 35 mg/L (as boron) registered concentration established by the U.S. EPA (2008b; 2015). However, the margin of exposure for children ages 7 to 10 calculated by the U.S. EPA (2008b) for the 35 mg/L concentration was 150, which exceeded the LOC of 100. Therefore, higher application rate (35 mg/l × 1.5 = 52.5 mg/L or 52.5 ppm) could be viewed as acceptable.
Similarly in 2015, the U.S. EPA calculated a margin of exposure of 160 for children ages 6 to IO as compared to the LOC of 100, which again suggests that a higher application rate may be acceptable. Nevertheless, both U.S. EPA assessments (2008b; 2015) indicate that an application rate resulting in a concentration of 35 mg/L (as boron) is currently authorized under FIFRA.
Assessment of boron under NSF/ANSI 50 also determined that the currently recommended application rates of boron (resulting in concentrations of 35-50 mg/L) did not pose a substantial risk to human health and this exposure has an LOC of 150 so you could use 1.5 times that amount with a margin of safety of 100 times. Our recommendation stands at 50 ppm borate for pools and spas. EPA says maximum CYA in pools is 100 ppm and how many pool are more than that?
 Borate Legal For Sale in California Since December 2017
Borate Legal For Sale in California Since December 2017
One maker of borate products has received this communication. “We have just received notification from California that the “Data Gap” that prompted the restriction on sales of Borates in California has been resolved to their satisfaction.
Going forward there are no current regulatory restrictions on these products being sold in California.” This communication was dated November 28, 2017. So if someone has told you borates are illegal in California, they are wrong.
Hope this helps you understand Borate.




3 Comments
Leave your reply.