Lately I have been hearing a lot of service techs asking where they can buy borate or boric acid because they heard it is a good thing.
By Robert W. Lowry
While it is true that just adding borate or boric acid to your pool will certainly help, there is a whole method that will make the pool stable and water conditions will change only a little from week to week.
The method involves adjusting water conditions to specific targets, stopping the use of trichlor, having a maximum CYA of 50 ppm, adding 50 ppm borate, only using liquid chlorine or cal hypo and having a chlorine level that is 5% of CYA. This method is for residential pools only.
The first step is to bring the water conditions to their targets:
pH 7.5
TA 90 ppm
CH 350 ppm
CYA 50 ppm max.
TDS 1,500 ppm + starting max.
Borate 50 ppm
Of course, if CYA or hardness are greater than the targets, you should drain some water and get them near the targets. Then you will have to re-test the water and bring the other water conditions to their targets.
The purpose of this is to make the water conditions stable. There are two buffers in the water that prevent or slow down the pH from going down. They are total alkalinity and CYA. There is one buffer that prevents or slows down the pH from going up. It is borate. Having TA at 90 ppm and CYA at 50 ppm will provide the right buffering for pH decreases. Having 50 ppm of borate in the water will prevent or slow down pH increases.
Next, stop using trichlor because it has a lot of CYA in it. In fact, for each 10 ppm of free chlorine provided by trichlor, 6 ppm of CYA is added. The maximum CYA is 50 ppm so if you are using trichlor and the chlorine use in a pool is 10 ppm of free chlorine per week, then the CYA will go up by 6 ppm per week or about 25 ppm per month. With a maximum of 50 ppm, you would get to the max in 2 months but that is only if you started at zero. In addition, trichlor is acidic and lowers pH and TA.
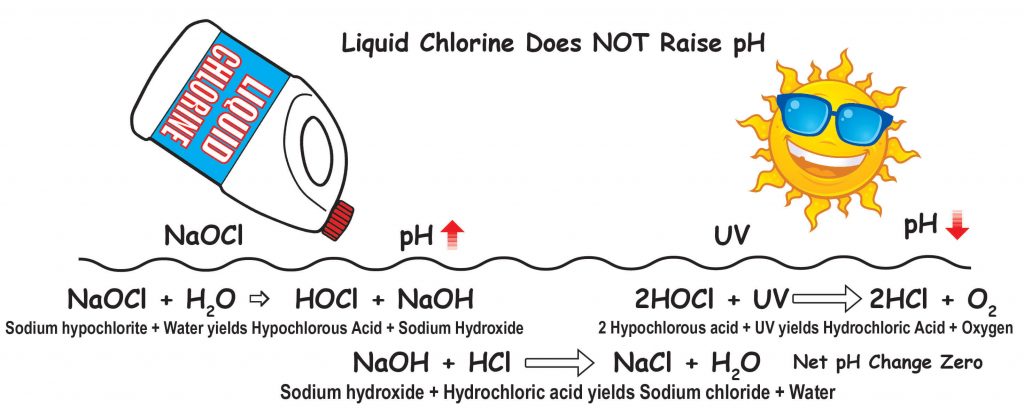
If you use liquid chlorine or cal hypo they do not have CYA in them so the CYA will not be increasing. Liquid chlorine will not raise the pH of the pool water. Many people are finally understanding this.
When sodium hypochlorite (liquid chlorine) which has a pH of 11 to 13 is added to the water, HOCl (hypochlorous acid) and NaOH (sodium hydroxide) are produced. The sodium hydroxide raises pH. HOCl is the killing form of chlorine in water. However, when HOCl is degraded by UV (sunlight) or used to kill organisms or oxidize anything, the HOCl becomes HCl. HCl is muriatic or hydrochloric acid. The amount of HCl made by HOCl being degraded is almost equal to the amount of hydroxide produced when the liquid chlorine was added. So the net result is that the pH change is zero or near zero. The amount of chlorine you need in a residential pool with borate at 50 ppm is 5% of CYA. The normal recommendation is 7.5% of CYA when borate is not present, but because borate is also an algaestat (not an algaecide), less free chlorine is needed
 The chlorine needed in a residential pool can be calculated. It takes 0.05 ppm HOCl to kill algae which are more difficult to kill than bacteria. When there is 30 ppm of CYA in the water, 97% of all the chlorine is bound to cyanuric acid – only 3% of all the chlorine is available for killing and oxidation. And if you remember, chlorine exists in water as HOCl and OCl– (hypochlorite ion). OCl– is the non-killing form of chlorine in water. The amount of HOCl and OCl– in the water is determined by the pH. At a pH of 7.5 there is roughly 50% HOCl and 50% OCl–. This means that only 1.5% of all the chlorine in the water is HOCl.
The chlorine needed in a residential pool can be calculated. It takes 0.05 ppm HOCl to kill algae which are more difficult to kill than bacteria. When there is 30 ppm of CYA in the water, 97% of all the chlorine is bound to cyanuric acid – only 3% of all the chlorine is available for killing and oxidation. And if you remember, chlorine exists in water as HOCl and OCl– (hypochlorite ion). OCl– is the non-killing form of chlorine in water. The amount of HOCl and OCl– in the water is determined by the pH. At a pH of 7.5 there is roughly 50% HOCl and 50% OCl–. This means that only 1.5% of all the chlorine in the water is HOCl.
But now we can calculate how much HOCl we have in the water. We multiply 1.5% or 0.015 times the free chlorine read- ing. For example if there is 2.0 ppm FC, we multiply 0.015 times 2.0 and get 0.03 ppm HOCl. This is below the 0.05 ppm HOCl needed to kill algae. Now try 3.0 ppm FC. 3.0 × 0.015 = 0.045 ppm HOCl. This is almost enough to kill algae. And finally, if we use 4.0 ppm FC we get 4.0 × 0.015 = 0.06 ppm HOCl which is enough to kill algae.
But rather than do this calculation each time we can just use 7.5% of CYA. So your target chlorine level in a pool without borate is 7.5% of CYA. But if you are going to use borate at 50 ppm, then the 7.5% is lowered to 5%. So if you have 50 ppm CYA, multiply 5% times CYA to get the Target free chlorine 50 × 0.05 = 2.5 ppm FC.
In summary, this method makes pool water stable by having TA, CYA and borate as buffers so the pH will not change. The pH will not be changing due to liquid chlorine. The CYA will not be changing due to liquid chlorine either. Trichlor will not be changing the CYA, pH, TA or the requirement for free chlorine. And calcium hardness is at the right level to have an almost perfect Saturation Index.
You may need a little acid or bicarb each week and add enough chlorine to last a week or use a liquid chlorine feeder or leave some chlorine with the pool owner or use cal hypo tabs or add a chlorine generator but nothing will be changing much from week to week.
The finer points of understanding this method are in the book Pool Chemistry for Service Pros.






2 Comments
Leave your reply.