So if I am using liquid chlorine and it does not raise pH, what is causing the pH to go up all the time?
The short answer is that you have the wrong total alkalinity (TA) or the TA is too high.
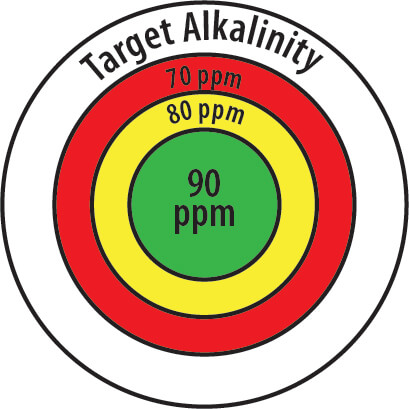
Try Using a Target of 90 ppm for TA
We recommend 90 ppm as a starting Target TA. If at this target the pH is drifting up or constantly rising, then change the Target to 10 ppm less or 80 ppm. And if that doesn’t work, try 70 ppm TA. If on the other hand your pH is drifting low or constantly going lower, raise the Target TA to 100 ppm. Again, change in increments of 10 ppm until the pH is stable.
 The problem is when the TA level is too high there is a lot of out-gassing of carbon dioxide (CO2) and that causes the pH to rise. You may also have a lot of splashing and aeration in the pool from waterfalls, spill overs, negative edge or other sources that accelerates the out-gassing. If you were to lower the TA to 80 ppm or even a little lower to 70 ppm, you will find that the rate of pH rise will be slower, especially if you target a pH of 7.5 and even slower with a pH of 7.6. If you find that a lower TA level helps, then you may need to raise the Calcium Hardness (CH) level a little bit to compensate for the saturation index to protect plaster surfaces. About a 50 to 70 ppm calcium increase should compensate nicely if using 70 ppm TA and 7.6 pH.
The problem is when the TA level is too high there is a lot of out-gassing of carbon dioxide (CO2) and that causes the pH to rise. You may also have a lot of splashing and aeration in the pool from waterfalls, spill overs, negative edge or other sources that accelerates the out-gassing. If you were to lower the TA to 80 ppm or even a little lower to 70 ppm, you will find that the rate of pH rise will be slower, especially if you target a pH of 7.5 and even slower with a pH of 7.6. If you find that a lower TA level helps, then you may need to raise the Calcium Hardness (CH) level a little bit to compensate for the saturation index to protect plaster surfaces. About a 50 to 70 ppm calcium increase should compensate nicely if using 70 ppm TA and 7.6 pH.
Aeration of the water, turbulence and splashing will raise the pH theoretically until the amount of carbon dioxide in the water is in equilibrium with the air. This pH of equilibrium then depends on the TA level. At roughly 200 ppm TA (ignoring CYA), the pH could theoretically rise to 8.77, but in practice you will see it on go up to about 8.5 or less. A TA of 100 ppm could have a max pH of near 8.0.
You can see that pools are way over-carbonated. We are just fortunate that the rate of out-gassing of CO2 is relatively slow and that even aeration and turbulence have a limited effect on speeding it up. If you want to run your aeration and turbulence-caus- ing systems all the time, then you will likely be adding acid all of the time. You might consider using a timer for aeration and turbulence-causing devices so that they run when people are around to enjoy them and not when people are at work or no one is there.
CO2 out-gassing is reduced by lowering TA, reducing turbulence and splashing, and keeping a slightly higher pH
The graphic below shows that CO2 in the atmosphere is in equilibrium with CO2 dissolved in the water.
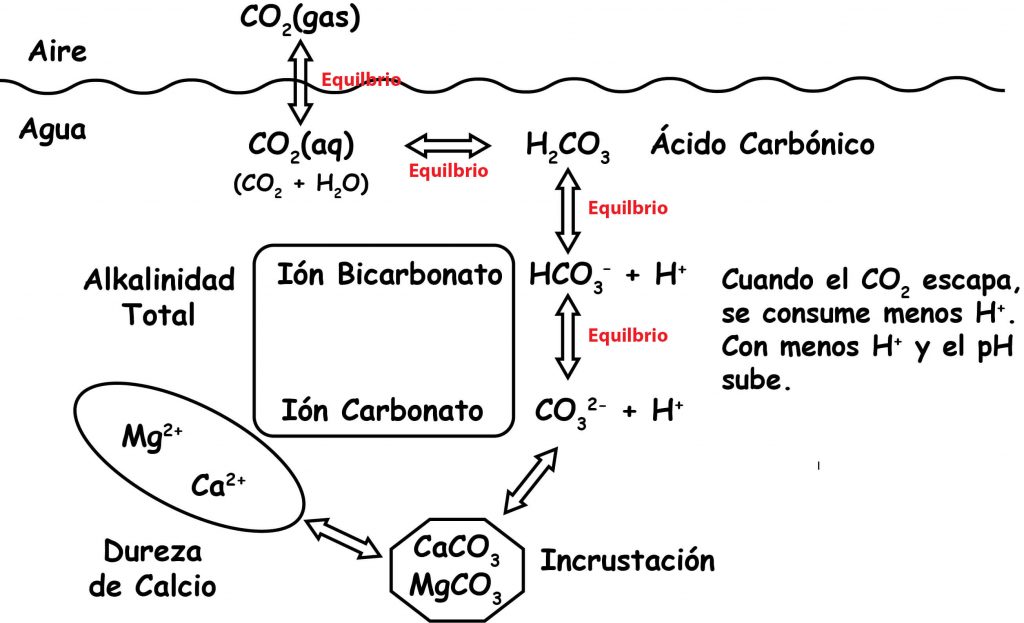 It also shows that CO2 in in equilibrium with H2CO3, and that H2CO3 is in equilibrium with HCO3-, and that HCO3- is in equilibrium with CO3 2–. The double arrows indicate that chemicals are in equilibrium and go back and forth to maintain equilibrium. So if you raise or lower any of these items, everything else shifts to keep the equilibrium.
It also shows that CO2 in in equilibrium with H2CO3, and that H2CO3 is in equilibrium with HCO3-, and that HCO3- is in equilibrium with CO3 2–. The double arrows indicate that chemicals are in equilibrium and go back and forth to maintain equilibrium. So if you raise or lower any of these items, everything else shifts to keep the equilibrium.
Adding aeration and turbulence forces CO2 out of the water and makes the pH go up. Adding CO2 makes the pH go down.
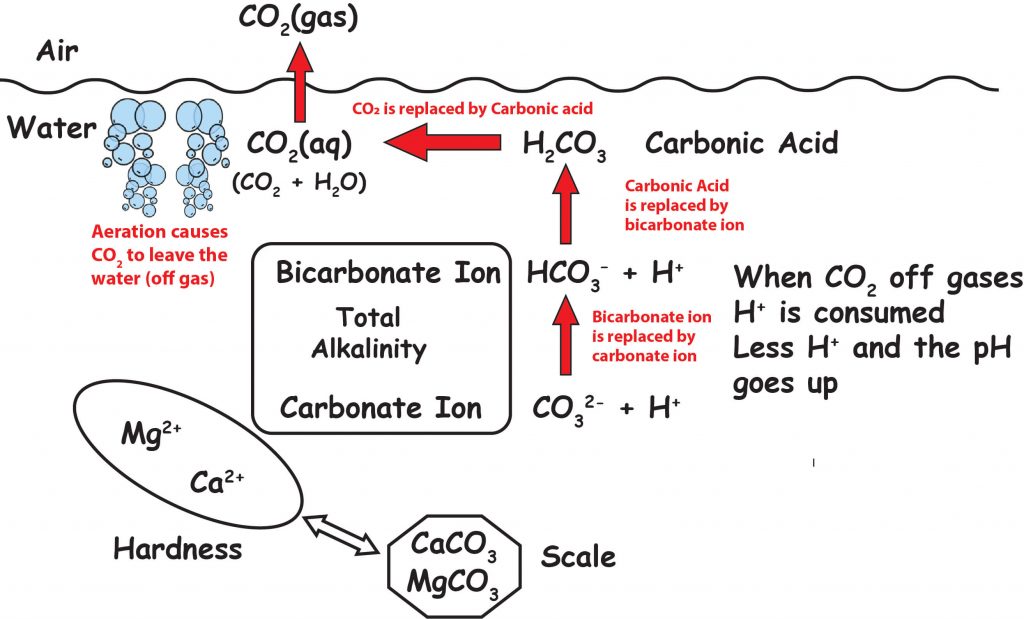 So if the pH is going up you now understand that it is most likely that the TA is too high, or there is a lot of aeration , splashing or turbulence. You are also thinking that the pH rise could be from liquid chlorine or bleach. This is logical thinking but even though liquid chlorine has a high pH, it does not raise pH. The pH rise could be from plaster curing or hydration. (But this only happens in newly plastered or new plaster pools.) The pH rise can also be from a SWG (salt water chlorine generator). The process of electrolytically making chlorine from salt added to the pool water causes the pH to rise because the hydrogen made is a gas and does not dissolve in the water and the SWG creates turbulence which removes CO2.
So if the pH is going up you now understand that it is most likely that the TA is too high, or there is a lot of aeration , splashing or turbulence. You are also thinking that the pH rise could be from liquid chlorine or bleach. This is logical thinking but even though liquid chlorine has a high pH, it does not raise pH. The pH rise could be from plaster curing or hydration. (But this only happens in newly plastered or new plaster pools.) The pH rise can also be from a SWG (salt water chlorine generator). The process of electrolytically making chlorine from salt added to the pool water causes the pH to rise because the hydrogen made is a gas and does not dissolve in the water and the SWG creates turbulence which removes CO2.
Liquid Chlorine and Bleach Have a High pH
Liquid chlorine and bleach (sodium hypochlorite) have a pH of 11.0 to nearly 13.0 so it is logical to think that they will raise the pH of the pool water. The fact is that initially or upon addition liquid chlorine raises pH because sodium hydroxide (lye) is made.
Here is what happens when liquid chlorine (sodium hypochlorite – NaOCl) is added to water:
NaOCl + H2O → HOCl + Na+ + OH–
sodium hypochlorite and water forms hypochlorous acid and sodium ion and hydroxide ion
You can see that hypochlorous acid (HOCl the killing form of chlorine) is made, sodium ions (Na+) and hydroxide (OH–) are made. The hydroxide raises the pH of the pool water. So your thinking was correct. Liquid chlorine raises the pH of pool water. But what happens next is something that I did not realize until a few years ago.
When HOCl is degraded by UV or sunlight, or used in the process of killing organisms or oxidation, the HOCl becomes HCl which is hydrochloric acid. The amount of acid made is almost equal to the amount of hydroxide that was made when the liquid chlorine was added. So the net difference to pH is zero or almost zero. Here is a graphic showing why liquid chlorine does not raise pool pH.
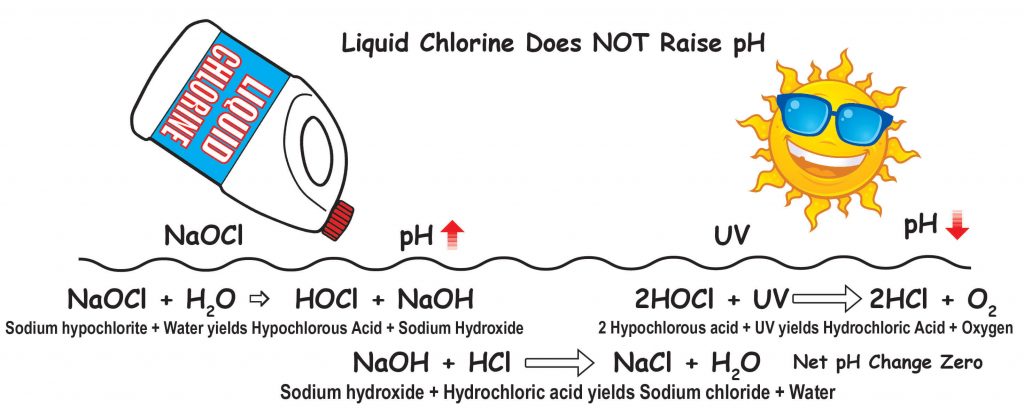
If I continuously chlorinate doesn’t that mean that the pH will always be high? The liquid chlorine raises pH initially because of OH (hydroxide) produced. But when HOCl degrades, it makes HCl and the amount of HCl is almost equal to the amount of OH. But then more chlorine is added and the pH goes up. Doesn’t the pH remain high?
You are correct that if you start with no chlorine in the water and you add hypochlorite then the addition will raise the pH and while it will get lowered as it is consumed or used, additional hypochlorite will raise pH again so that starting from 0 ppm and getting to and maintaining 3.0 ppm free chlorine results in an elevation of pH that is not lowered unless you explicitly lower the pH by adding acid.
But that is exactly what is done. Once the desired free chlorine level is reached, say 3.0 ppm, then the pH is lowered by adding acid. From that point forward, there is little change in pH from the hypochlorite itself except for the excess lye in it and that rise typically only shows up in some high bather-load pools using lots of chlorine per day.
The excess lye in liquid chlorine (sodium hypochlorite) is typically 0.03%. This is very little lye. Considering that a gallon of sodium hypochlorite 12.5% is 128 fl oz and weighs 9.66 lbs per gallon, there is about 0.046 oz (1.4 grams) of lye. This amount of lye would have a very small effect on pH in 15,000 gallons and that assumes you are adding the whole gallon of liquid chlorine to the pool.
So, again, if the pH is going up using continuous hypochlorite feed, the problem is high (wrong) TA, high aeration or turbulence. Start with a Target TA of 90 ppm and adjust up or down in 10 ppm increments until pH remains stable depending on whether the pH is drifting up or down. SWGs will always have the pH rising due to hydrogen off gas and turbulence created in the cell.
You should consider using borate at 50 ppm to help keep the pH from going up. It is an excellent pH buffer against pH increase.
For more information on using borate, please see the PCTI Tech Bulletin “Borate for Pools.”





3 Comments
Leave your reply.