Advanced Oxidation Process
AOP is an abbreviation for Advanced Oxidation Processes. In general, AOP is a group of chemical treatments and procedures designed to remove organic (and sometimes inorganic) matter in swimming pool water, spa water, drinking water, and wastewater by reactions with hydroxyl radicals (•OH).
In 1987, Glaze defined AOPs as “near ambient temperature and pressure water treatment processes which involve the generation of hydroxyl radicals in sufficient quantity to effect water purification.”
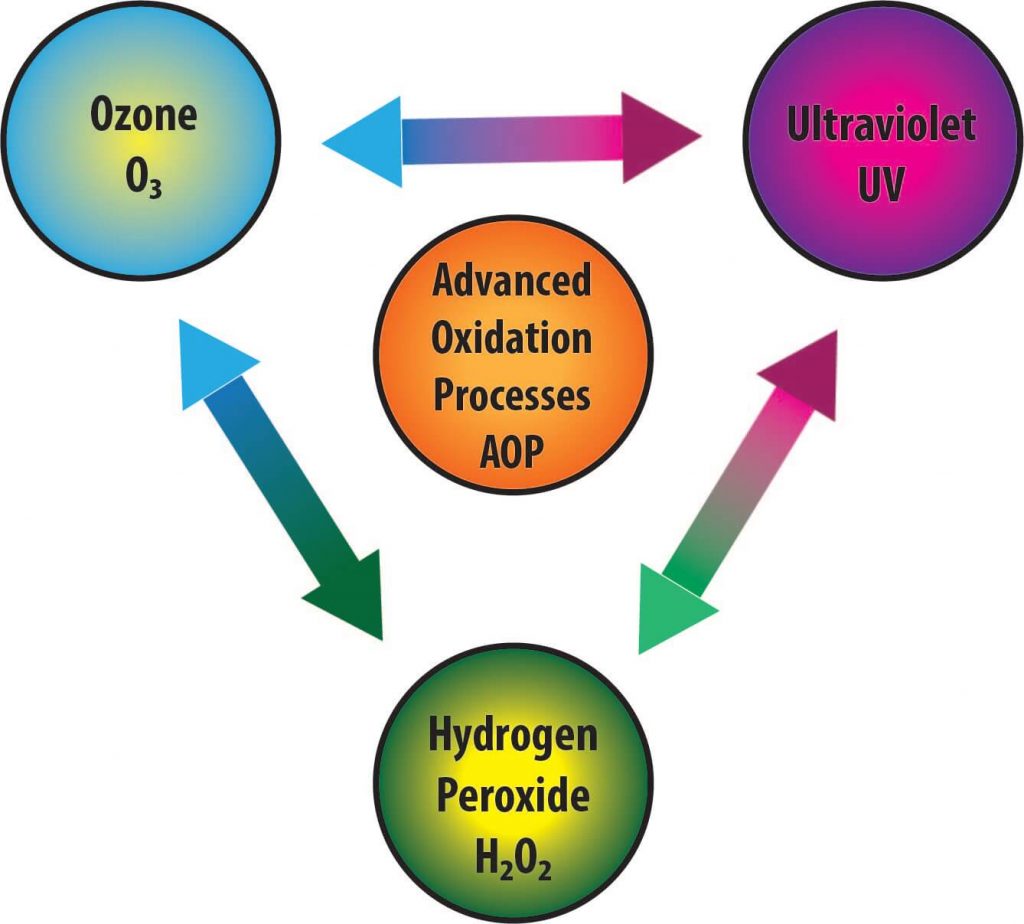
More specifically, AOP refers to any number of chemical or electrical processes that use a combination of ozone (O3), hydrogen peroxide (H2O2) and/or ultraviolet (UV) light to create or maximize production of hydroxyl radical (•OH) and remove organic matter.
AOP for Pools & Spas
- UV + O3 or (O2 in magnetic field)
- UV + H2O2
- UV + H2O2 + O3
In the graphic on the previous page, ozone (O3) directly oxidizes or destroys contaminants such as chloramines, combined chlorine, disinfection by products, organic materials, microorganisms and micro-contaminants. Ozone also decomposes into the much more powerful hydroxyl radical (•OH) and then the •OH rapidly oxidizes and decomposes organic materials.
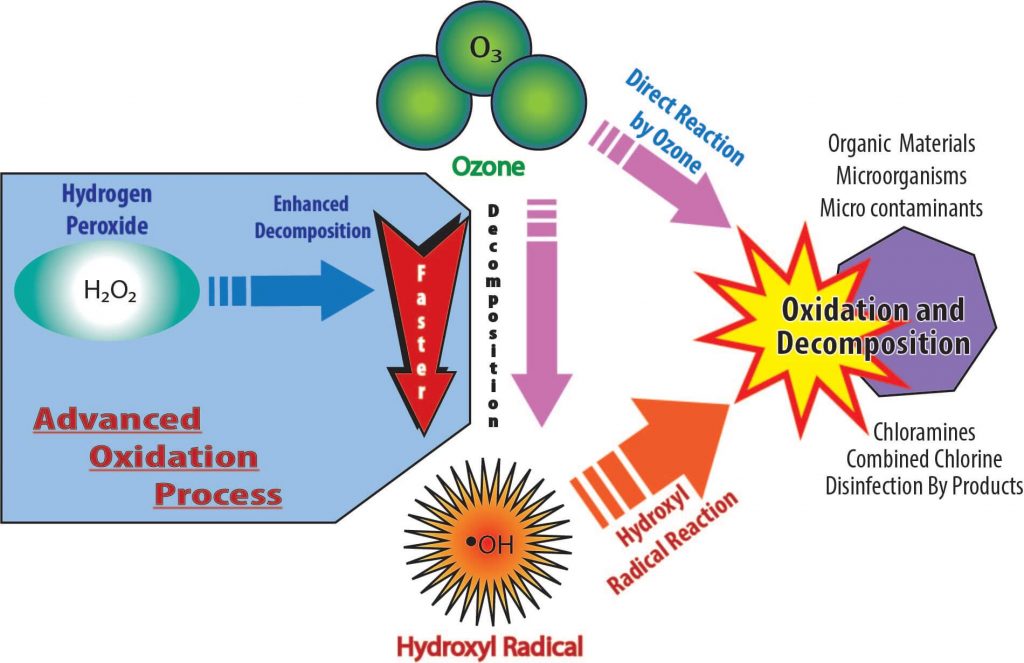 However, in the Advanced Oxidation Process (shown above in gray), hydrogen peroxide (H2O2) enhances the decomposition of ozone (O3) into hydroxyl radical (•OH). So the AOP makes radicals faster than normal ozone decomposition. This is shown graphically on the left above.
However, in the Advanced Oxidation Process (shown above in gray), hydrogen peroxide (H2O2) enhances the decomposition of ozone (O3) into hydroxyl radical (•OH). So the AOP makes radicals faster than normal ozone decomposition. This is shown graphically on the left above.
The goal of any AOP is to create or maximize production of •OH (hydroxyl radicals). All of these processes require equipment to produce the conditions that make the hydroxyl radicals. So it requires usually an ozonator and a UV sterilizer and/or the addition of hydrogen peroxide. (Although hydrogen peroxide is produced by some ozonators which eliminates the separate addition of hydrogen peroxide.)
AOP is Not New
Oxidation processes involving •OH have been around since the 19th century (Fenton Reaction 1894) however, the use of •OH for large-scale water treatment did not happen until 1987. Today there are about 500 commercialized AOP installations in the world for drinking water.
AOP is gaining a lot of popularity as droughts and water shortages force water treatment facilities to consider alternative sources of water and to consider wastewater recycling and treating wastewater so it may be re-introduced into receiving streams or the environment.
AOPs are applied primarily for destruction of organic or inorganic contaminants in water and wastewater. Although AOP inactivation of disease-causing organisms (called pathogens) and pathogenic indicators have been studied, AOPs are rarely employed for disinfection because these radicals have too short half-life (on the order of microseconds), so that the required detention times for disinfection are prohibitive due to extremely low radical concentrations. In addition, AOP does not produce a lasting or measurable residual in the water to provide protection against future introduction of pathogens or person to person contact. A low chlorine residual of about 0.5 to 1.0 ppm is recommended for this protection. And it could be more depending on bather loads and weather conditions.
All of the killing of organisms and destruction of organic and inorganic contaminants is done in the AOP chamber or within a few inches of the injection point. As a result, many AOP devices must be run 24 hours a day, every day. And even this does not guaran- tee safe water because if a biofilm or an algae colony develops on the pool walls, bottom or in the plumbing system, the ozone, UV and radicals produced are plumbed in-line and never get circulated in the pool.
•OH
AOP which produces •OH (hydroxyl radical) is a stronger, better and more efficient oxidizer than the weak form of chlorine in water (OCl– ,hypochlorite ion), the killing form of chlorine (HOCl – hypochlorous acid), UV (ultraviolet light) or O3 (ozone).
 The hydroxyl radicals are very reactive, unstable and have a very short half-life (about 1 nanosecond up to 10 microseconds). This causes •OH to react non-selectively and directly with dissolved solids. Harmful and toxic substances are decomposed to less harmful substances or even completely mineralized in water to carbon, nitrogen, hydrogen and oxygen. In short, •OH will destroy chloramines, disinfection byproducts, sweat, urine, sun block and all those organics that chlorine and non-chlorine oxidizers are used for. And they are so powerful that the destruction is practically instant. Think of this as a molecular atomic bomb. AOP also destroys bacteria, algae, virus and even parasites like Cryptosporidium parvum and Giardia lamblia. The bad news is that •OH is only around for 10 microseconds or less. There is no way to build up a residual and no practical way to measure it.
The hydroxyl radicals are very reactive, unstable and have a very short half-life (about 1 nanosecond up to 10 microseconds). This causes •OH to react non-selectively and directly with dissolved solids. Harmful and toxic substances are decomposed to less harmful substances or even completely mineralized in water to carbon, nitrogen, hydrogen and oxygen. In short, •OH will destroy chloramines, disinfection byproducts, sweat, urine, sun block and all those organics that chlorine and non-chlorine oxidizers are used for. And they are so powerful that the destruction is practically instant. Think of this as a molecular atomic bomb. AOP also destroys bacteria, algae, virus and even parasites like Cryptosporidium parvum and Giardia lamblia. The bad news is that •OH is only around for 10 microseconds or less. There is no way to build up a residual and no practical way to measure it.
 Making Hydroxyl Radicals (•OH)
Making Hydroxyl Radicals (•OH)
Some AOP’s use ozone combined with hydrogen peroxide (H2O2). This requires the addition of H2O2 which is a liquid. The ratio of H2O2 to ozone is important because the proper ratio enhances the production of •OH. UV light and H2O2 can also be used to
produce •OH. And finally, ozone, UV and H2O2 can be used together. Other devices use electrolysis to generate chlorine and •OH.
Some AOP devices have an ozonator and UV lamps in a single device that optimize the production of hydrogen peroxide so it does not have to be added separately. Basically everything is in one device.
The Chemistry of Making •OH
The reaction to make •OH is as follows:
3O3 + H2O2 → 2•OH + 4O2
AOP is an inefficient process. Carbonate and bicarbonate act as scavengers for •OH. They are total alkalinity in pool and spa water. The AOP process will be less efficient at 80 or 100 ppm total alkalinity than it is at 60 ppm. This complicates sizing of the AOP unit.
In the presence of other oxidants or irradiation, the •OH yield can be significantly improved. For example, in the so-called peroxone (O3/H2O2) system, the O3 decomposition and •OH production are enhanced by hydroperoxide (HO −) produced from H2O2 decomposition.
H2O2 → HO2− + H+
HO2− + O3 → •OH + O2−
In the O3/ultraviolet (UV) irradiation, H2O2 is generated as an additional oxidant primarily through O3 photolysis
O3 + H2O + uv → H2O2 + O2
H2O2+ uv → 2•OH
As a result, •OH can be generated, at a minimum, through the three pathways above. Ozone is combined with hydrogen peroxide
Hydrogen peroxide splits in water to the following reactions
H2O2 → HO2 + H
The HO2 (which is called hydroperoxyl radical,) reacts with ozone causing hydroxyl radical production.
2O3 + HO2 → 2•OH + 3O2
The activation of •OH is a very complex process which takes place according to a variety of different reaction mechanisms. The above reaction sequence is the reaction most often used for the complex creation of •OH (hydroxyl radicals).
The breakdown of chlorine by sunlight produces hydroxyl radicals according to these reactions.
HOCl + hv → OH• + Cl• and
OCl– + hv → O•- + Cl•
O•- + H2O → OH• + OH–
According to Richard Falk, Chem Geek at Trouble Free Pools, at a roughly 10% Free Chlorine to CYA ratio with roughly 0.05 ppm HOCl and OCl– at pH 7.5 the loss rate to UV from direct noontime sun is 0.12 ppm per hour throughout the entire pool exposed to sunlight. For an 18,000 gallon pool (68,137 liters) that is 8.2 grams Cl2 equivalent weight or 2.0 grams OH• per hour. In weight equivalent of ozone, this is 5.5 grams O3 per hour which is much higher than the typical 0.5 to 1.0 g/hr typically used in residential pools.
In other words, chlorine breakdown from UV is a hydroxyl radical generator throughout the pool! Outdoor pools are already producing some hydroxyl radicals from sunlight and chlorine.
Getting Hydroxyl Radicals into the Water
In residential swimming pools an AOP device is placed in the plumbing line similar to an electronic chlorine generator (ECG) or an ozonator. The full flow of water goes through the AOP device. In commercial or public pools, the device can be in-line with full flow or a side stream.
In the side stream, an amount of the flow (usually about 25%) of the full flow is diverted through the AOP device. The devices most often have a contact or kill chamber where the hydroxyl radical contact with contaminants is maximized. Because •OH exists for 10 microseconds or less there is no danger of any •OH reaching the pool.
However, the half-life of ozone is influenced by pH and is less as pH increases. Ozone also reacts with chlorine and is not avail- able for •OH generation. But the half-life of ozone in most pool or spa water is about 5 minutes or maybe up to 10 minutes in water that has fewer organics or oxidizable material.
A side stream may not be the most efficient or best plan. The reason is that in a re- circulating system one turnover (a turnover is the time it takes for the pump to flow a volume of water equal to the gallons in the pool), will only filter 63% of the water.
The Law of Consecutive Dilution is important here. Water that is filtered is returned to the pool and mixed with the unfiltered water. Then this water goes through the pump and filter. So in 1 turnover of full flow, 63% of the water is filtered but with a side stream of 25% only about 1/4th of the water is going through the side stream. Even with a 24 hour per day run time you only get 63% of the water through the side stream. Therefore it takes almost 2 days for all the pool water to go through the side stream.
One turnover will produce water that is 63% filtered, 2 turnovers will have 85%, 3 turnovers 95 and 4 turnovers 98%. The same applies to water going through the AOP device.
The pool water goes through the AOP device before the filter or a side stream that bypasses the filter.
Comparing AOP with Ozone and UV
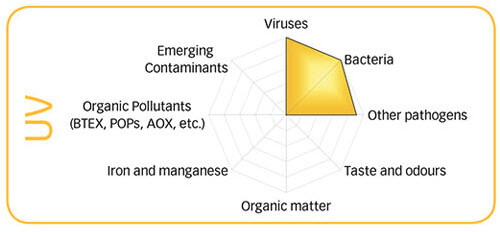
There is no question that AOP is the most powerful oxidizer that can be added to pool or spa water. UV is great at destroying viruses, bacteria and other pathogens like Cryptosporidium parvum and Giardia lamblia and E. coli. UV does not add anything to the water so there is no chemical change. However, UV is stationary and only contaminated water that passes through the UV light chamber is exposed to the UV rays. UV destroys chloramines and combined chlorine but does not do much against organics, organic pollutants, taste, odor, metals, biofilm or algae or anything that is growing in or attached to the vessel or plumbing.
According to Ernest R. (Chip) Blatchley “sometimes some organics with broken bonds are radicals that combine to form even worse com- pounds like cyanogen chloride.” And, of course, UV does not provide a residual in the water. UV may partially affect an organism and that organism may regrow or mutate.
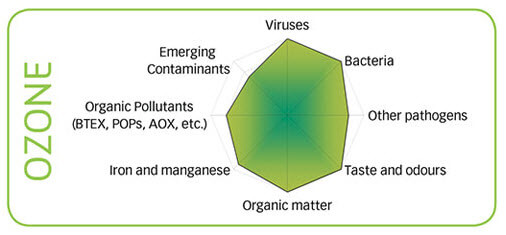
Ozone is very good at destroying virus, bacteria, taste and odor, chloramines and combined chlorine and most organic matter although it reacts slowly with ammonia. As you can see in the graphic, ozone does not cover all of the oxidizable things that can be in the water. Ozone has a short half-life in pool water from a few seconds for heavily contaminated water to a few minutes for water with not much oxidizer demand or with some amount of free chlorine in the water. It also does not provide a lasting residual and does nothing for biofilm, algae or other contaminants in the plumbing or on the pool walls or bottom.
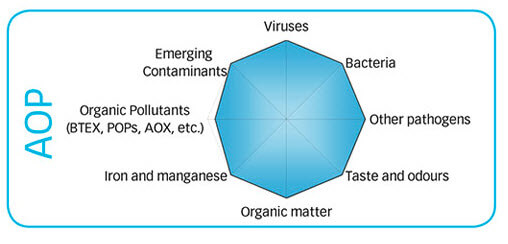
AOP covers almost all the possibilities of oxidizable things in the water and is an excellent disinfectant as you can see in the graphs to the right. However •OH is the shortest lived oxidizer with less than 1 second in water. Therefore it is very unlikely that it could ever be used as a stand-alone disinfectant and oxidizer and chlorine would be the best choice to use with it. Granted that you could probably reduce chlorine consumption by up to 90% if the AOP is sized properly.
Testing for Hydroxyl Radicals
In an analytical laboratory the presence of hydroxyl radicals can be verified by a benzoic acid chemical probe with thin-layer chromatography (TLC) and spectrophotometric wavelength scans. The presence of hydroxyl radicals is indirectly determined by detection of hydroxylated benzoic acids on TLC plates and a violet solution color with a peak absorbance at a wavelength close to 520 nm.
In 2007 a new test procedure was developed called the methylene blue dye test. It qualitatively indicates the presence of hydroxyl radicals through the immediate, distinct bleaching of methylene blue dye on a paper test strip. Bleaching of methylene blue dye, due to the presence of hydroxyl radicals in a sample, is indicated by a discoloration from a dark blue color to an almost white color and concentrated at the point of application, with a dark blue outline. A lack of bleaching indicates the absence of hydroxyl radicals in the sample.
So there is a test for •OH but you have to be quick about it because the •OH lasts for less than a second and you need to get the methylene blue test paper at the point of injection. This would not be an easy task in a pool with an in-line AOP.
So how do you know if it is working?
Well, you don’t. At least not by testing. The units have a light or some type of sight glass so you can see if the UV bulbs are on. But just knowing the bulb is on does not necessarily mean that you are making ozone or UV or creating hydrogen peroxide at the proper wave length or that you are making hydroxyl radicals.
The only way to figure out if an AOP device is working is to measure the chloramines or combined chlorine and see if by adding AOP the chloramines are lower or eliminated. Another way is to add an AOP device to the pool and lower the free chlorine residual until the AOP cannot kill or oxidize everything and the free chlorine level is being used. Then raise it slightly. You could also see how much longer a chlorine residual stays in the water before and after adding the AOP device.
•OH Dosage
There currently is no dosage level that is recommended.
In drinking water treatment they have the advantage of being able to test water before any treatment and after the treatment. As a result, they can make a test for a specific contaminant before the AOP treatment and make another test after the AOP treatment. In this way they can determine the effectiveness of the treatment.
So they might want to lower the level of PCE (perchloroethylene), TCE (trichloroethylene), atrazine, taste and odor compounds in the water. They make a test and determine that there is a level of 1.5 ppm atrazine before AOP treatment. Then they put some water through the AOP and measure atrazine after. If there is none, they can know that this was enough and maybe an excess of hydroxyl radical dose. If there still is some atrazine left in the water, they increase the AOP dose and try again.
In swimming pools and spas we recirculate the water in a closed system. It would be possible to check a before and after treatment for a specific contaminant . This is done currently by NSF for cryptosporidium. They measure crypto before and after to see that there is a 3-log reduction per pass. However, that is only for crypto. To determine AOP effectiveness would require selecting multiple organics and performing a test for each one. The organic testing may require expensive lab equipment such as gas chromatographs or HPLC. The treated water is mixing in the pool or spa with the untreated water. We can test for a specific contaminant and after some amount of time, see if the concentration of the contaminant is lower.
We might test the water and find out that we have 1.2 ppm of chloramine or combined chlorine. Combined chlorine is not a measure of a specific contaminant but a collection of amine-containing contaminants. The contaminants might be inorganic or organic – from chemicals or from people.
According to EPA, “Oxidation with molecular ozone occurs slowly in contrast to oxidation with hydroxyl radicals, which occurs very rapidly. Hydroxyl radical exposure is a function of temperature, pH, alkalinity, natural organic matter (NOM) concentration and composition. Hydroxyl radical exposure increases with increasing temperature, pH and NOM exposure (i.e., NOM decomposes ozone increasing the rate of hydroxyl radical formation), and decreases with increasing alkalinity (alkalinity scavenges ·•OH radicals). Some level of oxidation can occur naturally during ozonation (without peroxide addition); however, in O3/ H2O2 systems, hydroxyl radical exposure is significantly higher (100 times greater) than during conventional ozonation.” Taste and odor and organic micropollutants which are difficult to remove with ozonation alone.
The most efficient operational use of H2O2/ O3 is to add peroxide during the second stage of operation by injecting it into the second chamber of an ozone contactor. Oxidation with AOP is more reactive and faster and is effective in removing taste and odor and organic micropollutants which are difficult to remove with ozonation alone.
In some AOP, H2O2 is fed as an aqueous solution, at H2O2 to ozone ratios between 0.2 and 3.0. The optimal ratio for removing most compounds ranges from 0.3 to 0.6. The specific ratio is a function of disinfection requirements, bromide concentration, contaminant concentration, and other water quality parameters. Both ozone and AOP processes form bromate in the presence of bromide. (Bromates are cancer causing.)
Confusion over Dose and Output Amounts
The dose level of AOP and the output of the device is a major problem for AOP manufacturers. They make an AOP device and they have some way of determining how well it works or how much oxidation is provided under some circumstances. But they don’t tell anyone what that is. As a potential user of their AOP device, you have to rely on their advice to size a unit for a given pool.
This is not an ideal situation. One manufacturer may say that you should buy this unit while another manufacturer may say you need their unit and it is cheaper. How do you know which device to purchase? You don’t.
The ozone segment of our industry went through a similar dosing and sizing problem in the early 1990s. No one knew how much ozone a pool or spa needed and manufacturers claimed varying output amounts but each were using different output set ups and different methods of testing the output. Then they all had different claims on dose amounts. It was confusing and purchasers were the ones to suffer either because they had the wrong dose and the unit was inadequate for their conditions or they had too much and paid for it. Finally they all agreed on a test method and a dose amount from 0.1 to 0.4 ppm based on flow. Then the playing field was level and everybody knew how much ozone was needed. Customers could compare output and dose amounts and select a product that matched their system with the benefits they wanted.
So some day we may be able to have some kind of standard output test method and some amount of hydroxyl radical dose level criteria. Until the AOP manufacturers agree to standard methods their industry will suffer and prospective clients will be losers.
Chlorine Elimination
Right up front, I do not believe that you will be able to eliminate chlorine completely by using any AOP device to make •OH. The most obvious reason is that UV, ozone and •OH while they are very powerful oxidizers and disinfectants, they do not build up in the water, they do not create a residual and they don’t protect against future contamination or react to increased demand. They produce a fixed amount of •OH which might be enough for some amount of contamination for bathers but put 10 people in a spa or have 20 kids jump in the backyard pool and the AOP device can’t handle the increase.
Every AOP manufacturer will claim that you will be able to reduce the chlorine use by some percentage from 30 to 90 % or even eliminate chlorine completely. In addition, they will also claim that you will eliminate chloramines or combined chlorine, have better water quality and if some of the really bad organisms like cryptosporidium, giardia or E. coli HO157 ever get in the pool water that AOP will destroy it. This is true in most cases but if you have a fecal accident by someone with cryptosporidium there is no way to determine when that water will be safe to swim in again . There are NSF certification standards so you can in fact calculate how many turnovers it takes for how much log reduction using ozone or UV. Perhaps someday there will be standards for AOP. In all fairness, no form of chlorine will do it either. But these devices are sold with salesmen claiming that AOP will handle anything and you can be sure the water is safe if you have one. Not necessarily true.
Speaking of Chlorine Elimination…
As you make know, hydrogen peroxide is a chlorine neutralizer or reducer and UV light destroys chlorine in water.
So an AOP device will make it harder to maintain a free chlorine residual because the AOP will be destroying the free chlorine. It’s a balance between using less chlorine by oxidation of organics that chlorine doesn’t need to oxidize vs. the AOP residuals reacting with chlorine to consume it. As a general rule, high bather load situations with lots of organics will benefit from supplemental oxidation systems (AOPs) while low bather load will not. So some commercial/public pools will benefit, but most residential pools will not.
CO$T of Residential AOP Devices
It appears that the cost of a residential pool AOP device will be between $1,000 and about $3,000. Spa AOP units are less expensive.
Considerations
Pros
- Kills organisms like Cryptosporidium parvum and Giardia lamblia
- Destroys combined chlorine, chloramine, oxidation by products, organics, microorganism and micro contaminants
- Many times more powerful than chlorine
- Does not add any lasting chemicals to the water
- Reduces chlorine consumption from 50 to 90 % depending on device and conditions
- Provides better water quality
Cons
- Hydroxyl radicals (•OH) last for 10 microseconds or less
- Provides no residual in the water
- No way to verify it is working at optimum level
- All oxidation and killing is done in AOP device – no control of biofilm or algae
- Provides a fixed amount of ozone, UV, hydrogen peroxide and •OH – may not be adequate when bather loads are high
- May not provide protection against future introduction of pathogens or person to person contact
- Sometimes sold as chlorine free but not approved for that use
- No test for •OH or AOP
- No AOP or •OH dose guidelines or approvals
- Efficiency affected by high humidity, temperature, high pH, high alkalinity, iron, copper and manganese
- UV protective quartz sleeve needs cleaning frequently
- Replacement or consumable parts availability and cost
- Expensive







Leave a Reply
Your email is safe with us.
You must be logged in to post a comment.