PHTA Guideline and Health Codes May Be Inadequate
The PHTA Guideline for residential pools (ANSI/APSP-5 2011) states that the free chlorine level should be between 2.0 and 4.0 ppm. Many health codes have adopted this guideline. This may or may not be enough chlorine to control bacteria and algae in residential pools. We are talking about residential pools, not public or commercial pools. In residential pool the concern is controlling bacteria and algae. In public and commercial pools, the concerns are chloramines, DBP (disinfection byproducts) and oxidation of organic contaminants brought into the water by people. These organics may include sun block, deodorant, hair spray or gel, creams, urine, sweat, blood, mucous, skin particles, fecal matter and so on. These are introduced in residential pools also but usually there are a few people and thousands of gallons of water. Not so in public and commercial pools. So the main concern in virtually all residential pools is controlling or killing bacteria and algae.
 Bacteria and Algae Grow Fast
Bacteria and Algae Grow Fast
Bacteria take about 15 to 60 minutes to double in population under ideal conditions. For instance, the growth rate for E. coli is 15-20 minutes. Algae can double in population in about 3 to 8 hours. To prevent uncontrolled growth, the kill rate must exceed the growth rate for bacteria or algae. Specifically, that means killing more than half of the bacteria or algae in the time that it takes bacteria or algae to double in population. It is more difficult to kill algae than bacteria. Therefore it stands to reason that if the algae are killed, the bacteria will also have been killed.
HOCl and OCl–
Before discussing how much free chlorine is needed let’s review what happens to chlorine when it is added to water. When chlorine in any form is added to water, a rapid reaction takes place that produces hypochlorous acid (HOCl).
Here is what happens when chlorine is added to water:
Cl2 + H2O → HOCl + HCl
chlorine and water forms hypochlorous acid and hydrochloric acid
A second reaction is nearly instantaneous: Hypochlorous acid (HOCl) produced by adding any type of chlorine to water dissociates (comes apart into its ions) according to the following equation:
HOCl ↔ H+ + OCl–
hypochlorous acid dissociates into hydrogen ion and hypochlorite ion
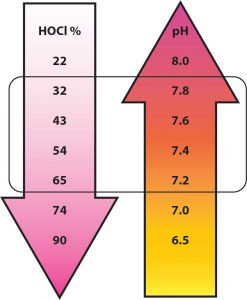
HOCl is the faster killing form of chlorine in water. OCl– kills but it is 30-300 times less effective than HOCl depending on conditions and organisms. The pH of the water determines how much of the free chlorine is in the HOCl and OCl– forms. When the pH of the water is low there is more HOCl. When the pH is high there is less HOCl. When the pH is 7.2, the HOCl is about 65% of the free chlorine. At a pH of 7.5 the HOCl is about 48% of free chlorine and at pH 7.8 the HOCl is about 32% of free chlorine.
It doesn’t really take much free chlorine to kill bacteria and algae. The World Health Organization (WHO) suggests the minimum HOCl for disinfection is 650 mV at a pH of 7.5 which corresponds roughly to about 0.01 to 0.05 ppm of HOCl depending on the ORP (oxidation reduction potential) instrument that is used. Killing green and black algae requires at least 0.02 to 0.03 ppm of HOCl. Therefore any amount of HOCl less than 0.01 ppm will not kill algae and an amount of between 0.01 and 0.050 ppm might kill algae. In any case, HOCl greater than 0.050 ppm will kill algae according to many sources. It Takes a Small Amount of HOCl to Kill Algae and Bacteria
Need 0.05 PPM HOCl to Kill Algae
Chlorine Binds to CYA and Slows Down Kill Times
When chlorine and CYA (cyanuric acid) are in the water, the chlorine binds to CYA and that is determined by chemical equilibrium. The slowdown in kill times is significant. HOCl, OCl– and all forms of chlorine bound to CYA together are free chlorine (free chlorine) and total chlorine. It is up to you to know how much of the chlorine that is in the water is in the killing form (HOCl). This requires knowing the pH and CYA level in addition to free chlorine.
Chlorine combines with CYA to form new chemicals called chlorinated isocyanurates. These new chemicals are not significant disinfectants or oxidizers. They are at least 150 times less effective. In other words, they don’t kill or oxidize much of anything. CYA has a strong affinity for chlorine so most of the chlorine in the water is bonded to CYA.
There are 6 different chlorine-CYA reaction compounds. The following is the primary relevant chemical equation:
Cl2C3N3O3– + H2O ↔ HOCl + H2C3N3O3–
chlorine bound to cyanuric acid and water forms hypochlorous acid and cyanurate ion
 Notice that there are two arrows. This is because the reaction can go in either direction (in equilibrium) depending on pH and other factors.
Notice that there are two arrows. This is because the reaction can go in either direction (in equilibrium) depending on pH and other factors.
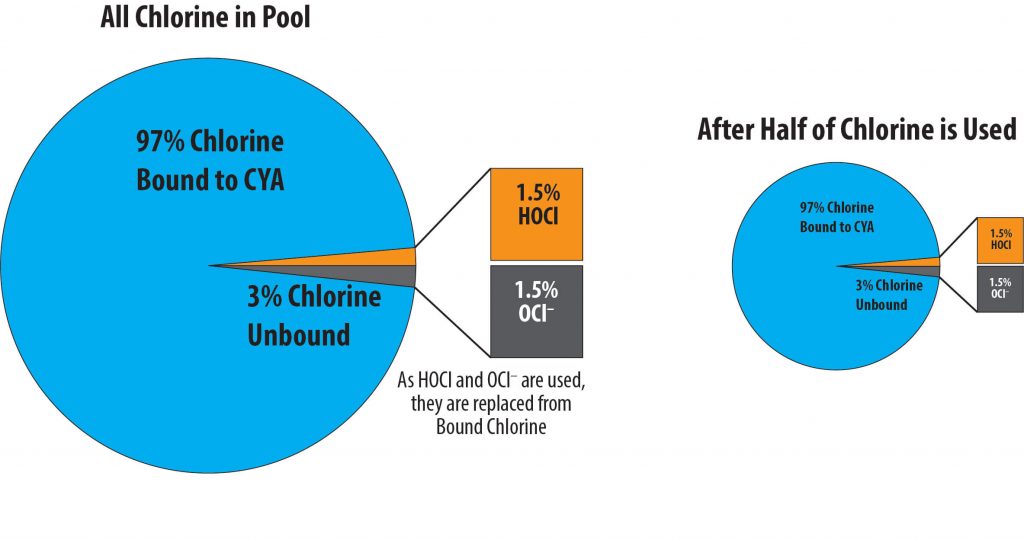
At a pH of 7.5 and a CYA of 30 ppm, 97.2% of the chlorine is bound to CYA. Therefore, with a pH of 7.5 there is only about 1.5% hypochlorous acid (HOCl), and 1.5% hypochlorite ion (OCl–) available for disinfecting and oxidizing.
Remember that at a pH of 7.5, there is about 50% HOCl and 50% OCl–.
Only 3% of All Chlorine in the Water is Available
50% is HOCl and 50% is OCl–
So ONLY 1.5% of ALL Chlorine in water is HOCl
Calculating the Amount of Free Chlorine Needed to Kill Algae
Based on the amount of chlorine bound to CYA and based on the pH of the water we can calculate if there is more than the 0.05 ppm of HOCl that is needed to kill algae in the water. There is only 1.5% or 0.015 times the free chlorine residual in the water.
2.0 ppm of Free Chlorine × 0.015 = 0.03 ppm HOCl (not enough Free chlorine to kill algae)
3.0 ppm of Free Chlorine × 0.015 = 0.45 ppm HOCl (maybe enough Free chlorine to kill algae)
4.0 ppm of Free Chlorine × 0.015 = 0.60 ppm HOCl (algae are killed)
3% of Chlorine not bound to CYA is in Equilibrium
Please understand that the amount of chlorine bound to CYA and the amounts as HOCl and OCl– are in equilibrium. This means that as some HOCl is used it is replaced by some OCl– and some chlorine that is bound to CYA becomes free chlorine. So if there is 1.5% of the free chlorine in the water as HOCl there will always be 1.5% until all the free chlorine is used. You can think of the amount of chlorine bound to CYA as a reservoir of free chlorine. We need to make sure that the 1.5% is enough to kill algae – greater than 0.05 ppm .
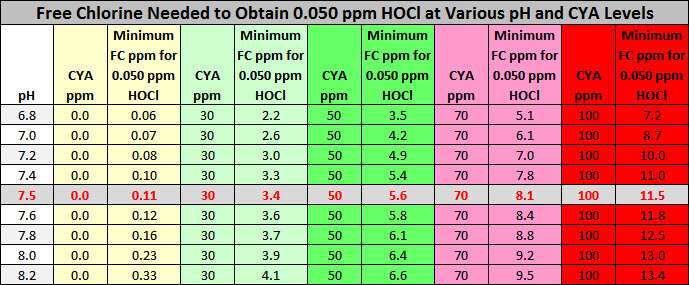
As you can see, pH 7.5 and CYA of 30 ppm, it takes 3.4 ppm free chlorine to obtain 0.05 ppm HOCl. This is nearly 11%. At pH 7.5 and 50 ppm CYA, it takes 5.6 ppm free chlorine to obtain 0.05 ppm HOCl. Again, this is nearly 11%. Rather than doing calculations or looking up conditions in charts, there is a better way. The free chlorine level 7.5% of CYA is a minimum.
Minimum Chlorine Needed to Obtain 0.05 ppm HOCl
Free Chlorine must be a minimum of 7.5% of the CYA level and only 5% of CYA if using borate at 50 ppm
If you have 50 ppm of CYA then you need 3.75 ppm Free chlorine minimum (50 × 0.075 = 3.75). If you have 100 ppm CYA then you need 7.5 ppm Free Chlorine (100 × 0.075 = 7.5 ppm). However, according to US EPA Guidelines, swimmers should not enter the water when free chlorine is more than 4.0 ppm. Therefore, the absolute maximum amount of CYA in residential pool water should be 50 ppm (50 × 0.075 = 3.75 ppm) to keep the free chlorine level below the EPA recommendation. However, if using borate at 50 ppm, the required free chlorine level is 5% and you could therefore have 80 ppm CYA because 5% of 80 ppm is 4.0 ppm.
Maintaining free chlorine at a minimum of 7.5% of CYA (or 5% of CYA if using 50 ppm borate) means you will not have to superchlorinate, add a separate oxidizer or use algaecides.
What If CYA Is Not Used?
Looking at the information on the chart on the previous page, you might be asking yourself what if I don’t use CYA. The answer is you could but it would be expensive and require that you add chlorine multiple times per day. The real world UV degradation of chlorine in a swimming pool is about 75% loss in 2 hours. The next 2 hours the loss is 75% of that. The chlorine that you added will be almost zero in 4 hours. Let’s say you start with 4.0 ppm Free chlorine. In 2 hours you lose 75% of 4.0 or about 3.0 ppm is gone. In the next 2 hours you lose 75% of 1.0 ppm or 0.75 ppm. Now you have only 0.25 ppm Free chlorine. In 4 hours you basically have no free chlorine in the water.
Also, it would be unrealistic to maintain 0.1 ppm free chlorine in a pool by using 1.0 ppm free chlorine with no CYA. At 1.0 ppm free chlorine, swimsuits, skin and hair are oxidized faster, equipment corrodes faster and chloramines are created faster.
CYA slows down the Free chlorine loss due to UV degradation from sunlight and significantly moderates chlorine’s strength. In fact, 30 ppm of CYA keeps chlorine in the water 8 times longer than without it.
EPA Chlorine Exposure Guideline Never Considered the Amount of Chlorine Bound to CYA
The EPA guideline for chlorine exposure is based on exposure to 4.0 ppm free chlorine when there is no CYA. EPA never considered the fact that 97.2% of the chlorine is bound to CYA at pH 7.5, 30 ppm CYA and 2.0 ppm free chlorine, or that 98.35% of the chlorine in the water is bound to CYA at pH 7.5, 30 ppm CYA and 4.0 ppm free chlorine.
So swimmer exposure to chlorine when using 30 ppm or more of CYA is only 1.5 to 2.8% of the free chlorine level in the water. For example, at 4.0 ppm free chlorine, swimmers are exposed to only 0.11 ppm chlorine (4.0 × 0.028
= 0.112). If there is 4.0 ppm of free chlorine with no CYA, swimmers are exposed to the whole 4.0 ppm. CYA keeps exposure to chlorine low. If using borate at 50 ppm, the free chlorine requirement is lowered to 5%. So you can have up to 80 ppm CYA because 5% of 80 is 4.0 ppm.
Trichlor Causes CYA Buildup and Algae
If you are using trichlor for disinfection then CYA will build up quickly because for each 10 ppm of free chlorine added to the water by trichlor, it also adds 6.0 ppm of CYA. Your CYA may be increasing by 6-8 ppm per week or 25-30 ppm per month.
If you don’t raise the free chlorine level as the CYA level increases, algae will begin growing. This is also the reason that during the summer a pool starts out with no algae and eventually it has algae. What is happening this. In the beginning of the swimming season you may have 30 ppm of CYA and keeping chlorine at 2.0-4.0 ppm. This is okay because 7.5% of 30 ppm means you need 2.25 ppm of free chlorine to prevent or kill algae.
In a month the CYA goes up by 30 ppm. Now you have 60 ppm CYA and you need 7.5% of CYA for free chlorine requirement so you now need 60 × 0.075 = 4.5 ppm free chlorine. If you are keeping 2.0 to 4.0 ppm free chlorine there is not sufficient chlorine to prevent algae. In another couple of weeks the CYA goes up by 15 and now you have 75 ppm CYA which means you need 5.6 ppm free chlorine to kill algae. So the recommended level of 2.0-4.0 ppm (and the EPA maximum) free chlorine is not enough to keep algae from growing.
Trichlor can be used as a sole chlorine source but you need to keep higher and higher levels of free chlorine as the CYA level goes up. One strategy is to use trichlor or a combination of liquid chlorine and trichlor until the CYA level is 50 ppm then switch to any source of chlorine without CYA (any hypochlorite – liquid chlorine or cal hypo).
Using Liquid Chlorine Does Not Raise pH or CYA
If you use liquid chlorine (sodium hypochlorite), liquid bleach (sodium hypochlorite), cal hypo (calcium hypochlorite) or lithium hypo (lithium hypochlorite) for regular chlorination, the CYA will not increase so you can keep a level of 30-50 ppm. Also, all of these hypochlorites have a net zero effect on pH.
If you use hypochlorite, the pH of the pool water will not go up except a small amount from the excess lye (sodium hydroxide) in the product to make the chlorine last longer in storage. The reason is that when you initially add one of these hypochlorites the pH goes up because the hypochlorite forms hypochlorous acid – HOCl and hydroxide (OH–). But when the hypochlorous acid is used killing bacteria or algae, used in oxidation of organics or destroyed by sunlight the reaction creates HCl (hydrochloric acid) which offsets the original rise in pH due to hydroxide except for the small excess of lye (sodium hydroxide) which does not change pH by a significant amount.
Lowering the Amount of Free chlorine Needed
There are ways to lower the free chlorine requirement of 7.5% of CYA. Anything that oxidizes or kills will lower the requirement. We have already stated that using 50 ppm borate in the water will lower the free chlorine requirement to 5% of CYA.
You could use a SWG (salt water chlorine generator) which replaces buying chlorine. Or you could use a supplemental device such as an ozonator, a UV sterilizer, a mineral purifier or a copper or silver ionizer. You could also add chemicals such as algaecides, phosphate removers, non-chlorine shock or oxidizers or enzymes. Depending on the supplements that are used, the required Free chlorine could be lowered to perhaps 2% of the CYA level but with a minimum free chlorine level of 2.0 ppm. With a SWG, the free chlorine recommended is 5% of CYA.
Testing Is Important
It should be clear that it is important to have accurate tests for pH, free chlorine and cyanuric acid. You must know that you have enough free chlorine (technically 0.05 ppm HOCl) to prevent algae growth. You can only know the proper amount of free chlorine if you know the cyanuric acid level and the pH.
Final Thoughts
Clearly keeping a free chlorine level of 2.0 to 4.0 ppm may or may not keep algae and bacteria from growing because it depends on the CYA level and the pH of the water. With the information in this PCTI Tech Bulletin you will be better prepared to manage the disinfection level in a pool to prevent algae and bacteria growth. Now you understand and have a way to know how much chlorine you need in any pool. It’s not the same for every pool.








Leave a Reply
Your email is safe with us.
You must be logged in to post a comment.